Abstract
Introduction: In a proportion of patients with myeloproliferative neoplasms (MPNs), severe remodelling of the bone marrow microenvironment develops, with myelofibrosis (MF) and osteosclerosis leading to cytopenias and poor survival. Mesenchymal stromal cells (MSCs) have been implicated in MF pathogenesis (Leimkühler, Cell Stem Cell, 2021), including Nestin+ (Arranz, Nature, 2014), Gli1+ (Schneider, Cell Stem Cell, 2017) and Leptin-receptor+ MSCs (Decker, Nat Cell Bio, 2017). However, little is known about the cellular subtypes and transcriptional states of fibroblast subtypes in MF, and the key receptor-ligand (R-L) interactions that lead to their activation. Our aim was to build a fully comprehensive atlas of MF bone marrow and clarify the pathological interactions between MPN cells and their stromal environment to determine the key drivers of inflammation and fibrosis.
Methods: We performed single cell RNA sequencing of mononuclear cells, hematopoietic stem/progenitor cells (HSPCs), CD41+ cells and CD45- Lin- Ter119- CD71low/- stromal cells from MF (MPLW515L) mice and controls, as well as CD45- Lin- CD235ab- CD71low/- stromal cells from MPN patients. Targets were validated using a novel human bone marrow organoid platform(Khan et al, bioRxiv, 2022) and by analysis of bone marrow biopsies from MPN patients and controls.
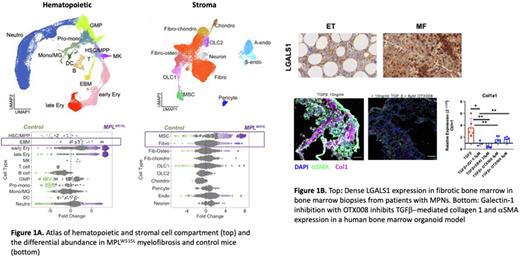
Results: More than 75,000 cells were analysed from control and MF mice, capturing 42,319 hematopoietic cells (including HSPCs, myeloid, lymphoid, megakaryocytes) and 34,969 stromal cells, including fibroblasts, MSCs, osteoblasts, chondrocytes, pericytes, arterial and sinusoidal endothelial cells (Fig.1A). Erythroid, neutrophil, megakaryocytes and also eosinophil-basophil-mast (EBM) cells were increased in abundance in MF mice, together with a ~4 - 8 fold expansion of MSCs and a more modest increase in overall abundance of fibroblasts. Extracellular matrix factors were expressed mainly by stromal cells (MSCs, fibroblasts) but also by EBM cells and neutrophils. As reported (Leimkühler, Cell Stem Cell, 2021), expression of hematopoiesis support factors by MSCs was reduced in MF. However, prior studies have not captured fibroblast subtypes. We found that the reduction in niche support from MSCs was compensated by a significant increase in hematopoiesis support factors from fibroblasts and EBM cells. Despite minimal increase in fibroblast cells overall, a subset of inflammatory fibroblasts (iFib) with high metabolic and glycolytic activity, TNFα signaling via NF-κB and TGFβ signaling was dramatically expanded in the MF mice, and also detectable in stromal cells from MPN patients.
This atlas enabled detailed study of aberrant cross-talk between hematopoietic and stromal compartments. Overall, receptor-ligand (R-L) interactions were 20% higher in MF mice due to increased signaling from megakaryocytes, EBM, MSC and iFib cells, implicating these four cellular subsets as key mediators of pathogenic cross-talk. R-L interaction analyses predicted increased TNFα signaling (TNFRSF1A → TNF) from EBM to both MSC and iFib, and increased TGFβ signaling (TGFBR2 → TGFB1) between EBM and megakaryocytes to MSC and fibroblast subtypes, including iFib.
The expanded subsets of megakaryocytes and EBM cells were noted to express notably high levels of galectins LGALS1 and LGALS3, encoding soluble β-galactoside-binding proteins associated with inflammation and tumorigenesis, and galectin signaling via predicted interacting partners was significantly increased in MF. High LGALS1 correlated with significantly shorter survival in patients with myeloid malignancies in the Cancer Genome Atlas myeloid leukemia cohort. Inhibition of galectin-1 with OTX008 significantly reduced TGFβ-induced collagen 1 and αSMA expression by human bone marrow stromal cells in 2D cultures as well as in human iPSC-derived bone marrow organoids, and bone marrow biopsies from a cohort of MF and non-fibrotic ET/PV patients showed a striking association of galectin-1 with fibrosis (Fig.1B).
Conclusions: We present a roadmap of the cellular and molecular landscape of normal and myelofibrotic bone marrow. We identify two cellular subsets (EBM and iFib) whose role as mediators of the inflammatory microenvironment in MF was previously unappreciated, and propose galectin-1 as a novel biomarker and potential mediator of fibrosis.
Disclosures
Sirinukunwattana:Ground Truth Labs: Consultancy. Mead:Galecto: Consultancy, Research Funding, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Sensyn: Consultancy, Speakers Bureau; Karyopharm: Consultancy, Speakers Bureau; Sierra Oncology: Consultancy, Speakers Bureau; CTI: Consultancy, Speakers Bureau; AbbVie: Consultancy, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Celgene/BMS: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Alethiomics Ltd: Consultancy, Current equity holder in private company, Other: Co-founder and equity holder, Research Funding. Psaila:Constellation Therapeutics: Consultancy; Blueprint Therapeutics: Consultancy; Novartis: Consultancy, Honoraria, Speakers Bureau; Galecto: Research Funding; Evotec: Research Funding; Alethiomics: Consultancy, Current equity holder in private company, Honoraria, Other: Co-founder , Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal